Kleiner Helfer, große Wirkung
Das Competence Center Schreiner LogiData steht für innovative, kunden-individuelle RFID-Entwicklungen. Doch jetzt haben die Experten einmal etwas für sich entworfen – wovon im Endeffekt natürlich
Behältermanagement mit RFID: Markierung mit Mehrwert
Als einer der weltweit führenden Hersteller hochwertiger Spritzgießmaschinen für die Kunststoffverarbeitung spielt für das Schwarzwälder Familienunternehmen ARBURG Produktionseffizienz eine große Rolle. Um diese weiter zu
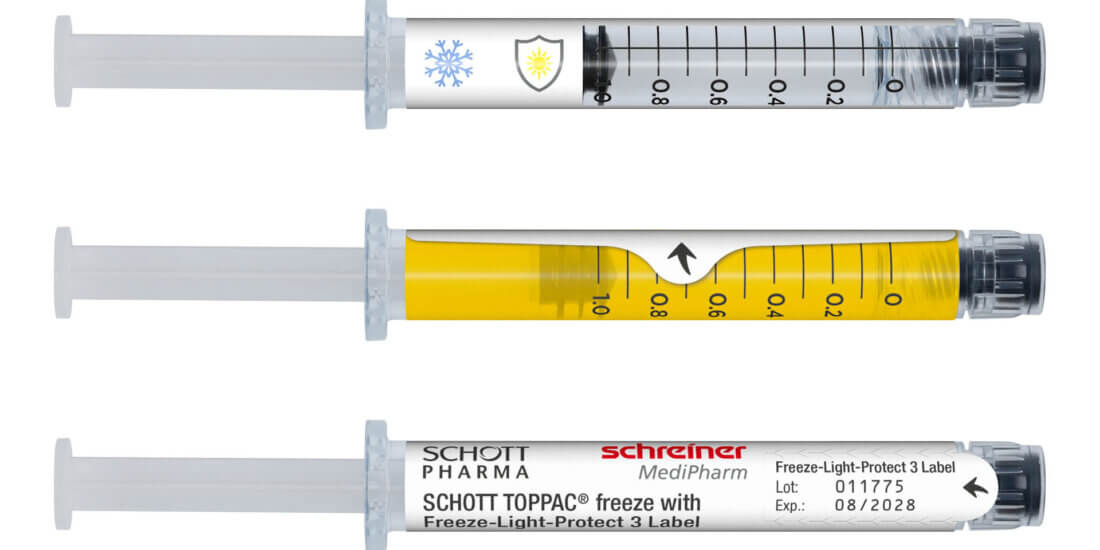
Therapiekontrolle: Digital und kindersicher
Mit den Smart Blister Wallets von Schreiner MediPharm kann die Therapietreue in klinischen Studien digital und in Echtzeit überwacht werden. Mithilfe seines Partners Keystone, einem
Erfolg durch Familienfreundlichkeit
Die Schreiner Group wurde 2023 erneut mit dem renommierten Award „Erfolgreich. Familienfreundlich“ ausgezeichnet. Das Familienunternehmen setzt mit flexiblen Arbeitszeitmodellen, Kinderbetreuung und weiteren unterstützenden Maßnahmen Standards
Hohe Investition in die Ausbildung
Eine hochwertige Ausbildung gilt bei der Schreiner Group als wichtiger Baustein, um jungen Menschen einen erfolgreichen Berufseinstieg zu ermöglichen und gleichzeitig dem Fachkräftemangel zu begegnen.
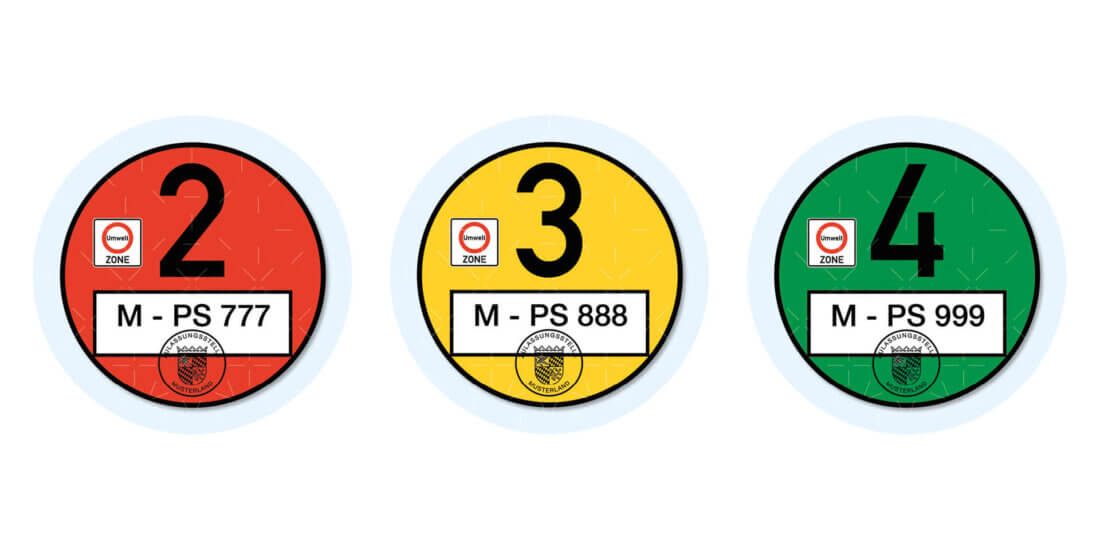
Freie Fahrt statt dicker Luft
Es gibt sie in Rot, Gelb, Grün und sie klebt in nahezu jedem Fahrzeug in Deutschland gut sichtbar an der Windschutzscheibe: Seit 2008 ist die
3rd License Plate Kennzeichen für den Kosovo
Die Verkehrssicherheit erhöhen, die Zahl der nicht registrierten Fahrzeuge verringern. Das sind die Hauptgründe dafür, dass die Behörden im Kosovo sich für die Einführung des
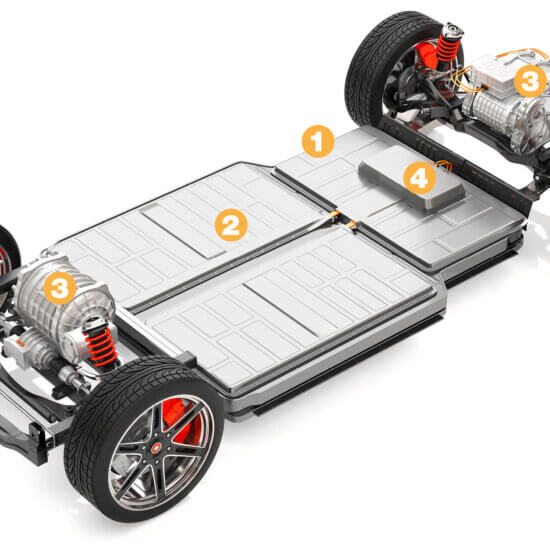
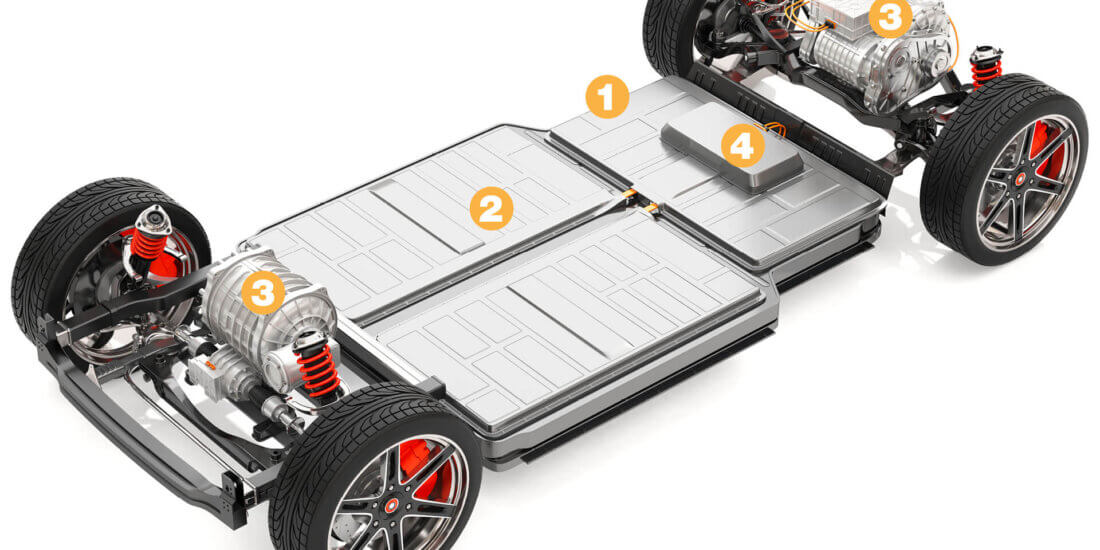
E-Mobility – Lösungen für den Megatrend
Elektroautos sind auf der Überholspur: Knapp 620.000 Fahrzeuge waren zum Jahresbeginn in Deutschland zugelassen, Tendenz steigend. Vom Druckausgleichselement bis zum Wallboxlabel – Schreiner ProTech bietet
Druckausgleichselemente zur Belüftung und Entlüftung
Durch Temperaturschwankungen verursachter Über- oder Unterdruck kann Gehäuse verformen und zu Undichtheit führen. Druckausgleichselemente von Schreiner ProTech sorgen für Druckausgleich und schützen Gehäuse und Komponenten.
Grüezi, Schweizer Vignette!
In der Schweiz ohne Vignette erwischt zu werden, ist teuer – kein Wunder: Bau, Betrieb und Unterhalt der Nationalstraßen verursachen hohe Kosten. Daher dient schon
Luer-Adapter-Fixierung: Sichere Verbindung für Spritzennadeln
Impfstoffe, Schmerzmittel oder Notfallmedikamente müssen intramuskulär injiziert werden, damit sie richtig wirken. Häufig werden dazu Luer-Lock-Spritzen eingesetzt, bei denen das medizinische Personal die Injektionsnadel mit
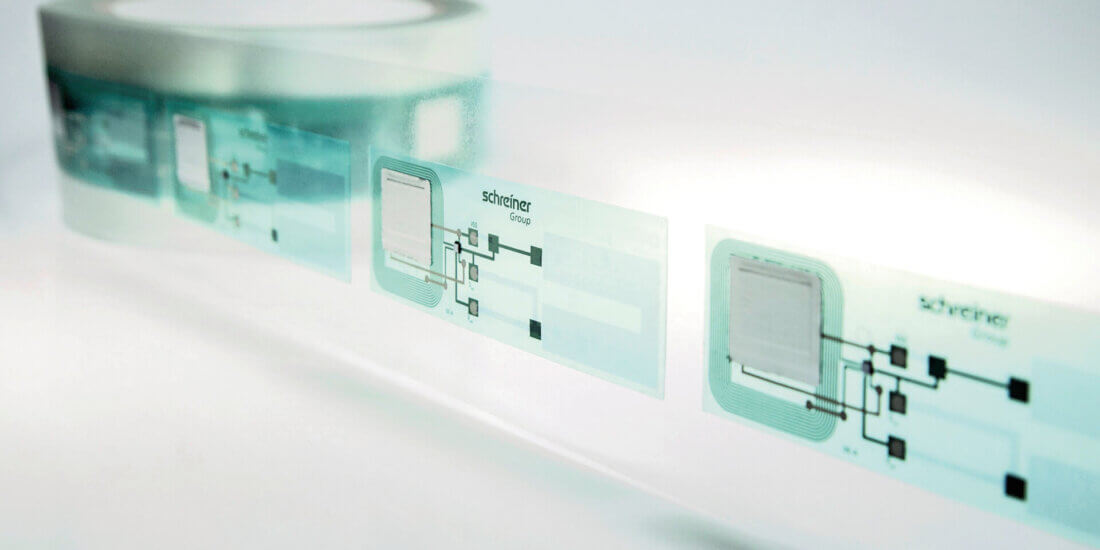
Die Welt der gedruckten Elektronik
Was ist gedruckte Elektronik? Welche Vorteile bietet gedruckte Elektronik? Was sind Entwicklungsthemen in der gedruckten Elektronik? Wie kann gedruckte mit konventioneller Elektronik verbunden werden? Welche
Beständige Kennzeichnung in rauer Umgebung
Im Maschinenbau müssen viele dauerhaft gekennzeichnete Komponenten einen Schutzanstrich erhalten – nicht nur in der Produktion, sondern auch später nach einer Reparatur. In beiden Fällen
Sicherheit garantiert: Neues PIN-Portfolio
Die Digitalisierung bringt viele Vorteile mit sich – dennoch sind manche Alltagsabläufe in analoger Form sicherer. Dazu zählt auch der Geheimnummernschutz, der per Brief verschickt